Investigations
The Bioglutide Scam: Lies and Fraud at Biomed Industries
In this article, I’m going to present multiple lines of evidence to explain why I think NA-931, marketed as Bioglutide, doesn’t exist, and that Lloyd L. Tran (CEO of Biomed Industries) has engaged in extensive scientific fraud and plagiarism. I have compelling reasons to believe that Mr. Tran’s primary motive for this fraudulent activity is to swindle investors. Attached to this blog post is a lengthy video outlining the facts: this post serves as a prophylactic repository of primary evidence to support the claims made in the video.
A GLP-1 Receptor Agonist Primer
Given my interest in GLP-1 receptor agonists (GLP-1 RAs) and peptide research, I spend quite a bit of time looking at pipeline drugs to assess what the future of the space might look like. You might be familiar with semaglutide (Ozempic, Wegovy and Rybelsus) or tirzepatide (Mounjaro and Zepbound), both are GLP-1 RAs. They help with diabetes, but in recent years, their main use has been as a medicine for weight loss.
Extreme competition exists between pharmaceutical companies researching GLP-1 RAs: if you’re able to find an effective drug that assists with weight loss and doesn’t have an extreme side effect profile, you have a license to print money. Right now, the best GLP-1 RA on the market is tirzepatide, but I suspect that when retatrutide is available in a few years, it will dominate the space.
Semaglutide was the first real GLP-1 RA that made sense to endorse as a first-line medication against obesity: although this class of drugs has existed for a few decades, prior iterations weren’t great. Semaglutide is good, but tirzepatide is a lot better – both in terms of weight loss and tolerability. As for retatrutide? It’s humanity’s greatest medical breakthrough in quite some time.
Enter Bioglutide
In February of 2024, an obscure medical company called Biomed Industries posted the following on their website (live, archived):
Biomed Industries, Inc. (Biomed) announced today that it has developed a promising obesity drug, NA-931, which is poised for Phase 2/3 clinical trials. NA-931 is a small molecule drug available in oral formulation…
… “We are actively seeking collaborators to work with us on this exciting new approach for the treatment of Diabetes and Obesity. NA-931 is novel in its approach to targeting both GLP-1 and IGF-1. The drug has shown only minor adverse events in a Phase 2A trial”, said Dr, Lloyd Tran, Chairman and CEO of Biomed Industries
And a press release archived from February 2024 on PR.com confirms that this claim was not added to the website at a later date:
16 months later, in a submission to the academic journal Diabetes, Lloyd L. Tran submitted preliminary topline results of NA-931 allegedly gathered from a Phase 2, 13-week randomized trial (live, archived):
The 13-week MAD study showed NA-931 demonstrated dose-dependent reductions in mean body weight from baseline, up to 14.8 % at 150 mg daily dosage, or 13.2% % [sic] relative to placebo…
…No muscle loss was observed. No clinically meaningful differences were reported for GI-related adverse events among subjects treated with NA-931 compared with placebo.
There are three things claimed about Bioglutide (the trademark name for NA-931) in this submission that are incredible if true.
- Mean body weight loss of 14.8% after 13 weeks
- No muscle loss, suggesting almost all weight loss would have been adipose tissue
- No clinically meaningful GI-related advertise events compared with placebo
Putting the first point into perspective, Mr. Tran claims that the clinical data gathered on NA-931 shows that it’s more effective at raw weight loss than retatrutide. Here’s a look at retatrutide’s data from the biggest study conducted thus far:
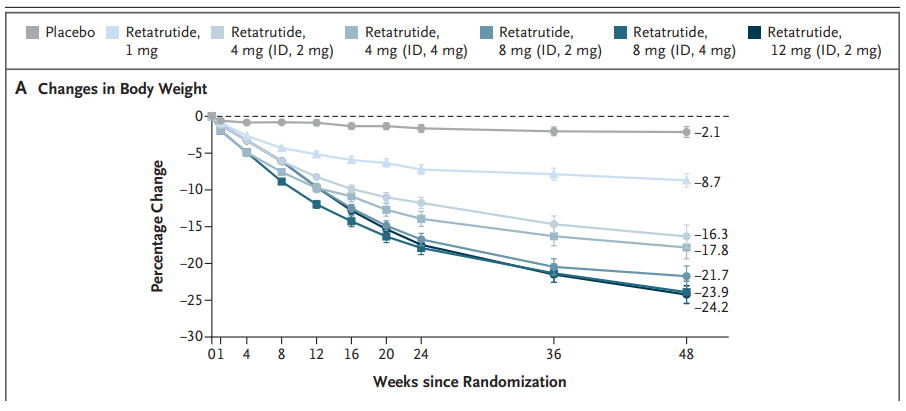 In the max-dose cohort, mean weight loss at 12 weeks was around 12.5% – a fantastic number, but Bioglutide is allegedly better. Additionally, addressing claim 2, all GLP-1 RAs tend to have somewhere around 25% lean mass loss, so we could reasonably assume that around 9.5% of the 12.5% retatrutide figure is non-lean mass (mostly adipose tissue), with the remaining 3% being lean mass (mostly muscle).
In the max-dose cohort, mean weight loss at 12 weeks was around 12.5% – a fantastic number, but Bioglutide is allegedly better. Additionally, addressing claim 2, all GLP-1 RAs tend to have somewhere around 25% lean mass loss, so we could reasonably assume that around 9.5% of the 12.5% retatrutide figure is non-lean mass (mostly adipose tissue), with the remaining 3% being lean mass (mostly muscle).
Extrapolating from these numbers, if the highest dose of retatrutide results in 9.5% non-lean mass loss and the highest dose of Bioglutide results in 14.8% non-lean mass loss, Bioglutide would appear to be 56% better than retatrutide in a key endpoint.
Finally, although the side effect profile of retatrutide is relatively minimal, the max-dose cohort had 15% of respondents reporting diarrhea and 19% reporting vomiting: well above the figures reported for Bioglutide by Mr. Tran in a press conference we’ll look at later.
If these claims are to be believed, Bioglutide is a drug unlike anything else we’ve seen before. Remember that retatrutide is already a candidate for drug of the century, and Biomed Industries claims that their drug is better in every respect.
Mr. Tran has also presented information about Biomed Industries – and the drugs it claims to have developed – at multiple conferences and events over the years.
I don’t believe that Bioglutide exists, but incredible clinical trial data isn’t anywhere near sufficient by itself to prove fraud beyond on a reasonable doubt. The rest of this article is going to point to multiple lines of evidence that cast doubt on the existence of the drug, the data associated with the drug, and will cover Mr. Tran’s history of fraud and lies.
Inconsistencies in Biomed Industries’ Story
Going back to the original announcement (live, archived) made by Biomed Industries in February of 2024, Mr. Tran was quoted as having said:
“The drug has shown only minor adverse events in a Phase 2A trial”, said Dr, Lloyd Tran, Chairman and CEO of Biomed Industries.
If you’re a US-based pharmaceutical company testing new drugs on humans, you’re required to lodge information about your clinical trials with the federal government at ClinicalTrials.gov. A search for NA-931 returns three results: one trial that hasn’t begun recruitment yet (NCT06732245), a 28-day Phase 1 safety and tolerability study (NCT06615700) and a 13-week Phase 2 trial in patients with obesity or overweight (NCT06564753).
If you’d like some additional (and more enjoyable) reading about participation in Phase 1 trials, Scott Alexander posted this from an anonymous Astral Codex Ten reader about the topic. To cut a long story short, Phase 1 trials test drugs for tolerability and safety. Quoting from the post I just linked:
I’m going to be focusing here on a research subject’s view of what are known as Phase I clinical trials, the stage in which prospective drugs are tested for safety and tolerability. This is where researchers aim to answer questions like “Does this drug have any dangerous side effects?” “Through what pathways is it removed from a patient’s body?” and “Can we actually give people enough of this drug that it’s useful for anything?” This comes before the stage where researchers test how good a drug is at actually treating any sort of disease, when patients who’re suffering from the target ailments are given the option receive it as an experimental treatment.
So basically, you do a Phase 1, you confirm it’s safe across various metrics, test dose responses, then you start a Phase 2 study – if and only if you’ve confirmed tolerability and safety. Conducting Phase 2 research before completion of Phase 1 research is unethical and dangerous. Why does this matter? Because of the dates.
Mr. Tran made statements about Bioglutide’s rates of adverse events from an alleged “Phase 2A trial” in February of 2024. However, if we look at the Phase 1 study submission lodged on ClinicalTrials.gov (NCT06615700), Biomed Industries claims that it began in May of 2024. How could Mr. Tran have known the adverse event rates from a Phase 2 trial on Bioglutide three months prior to Bioglutide’s Phase 1 study even beginning?
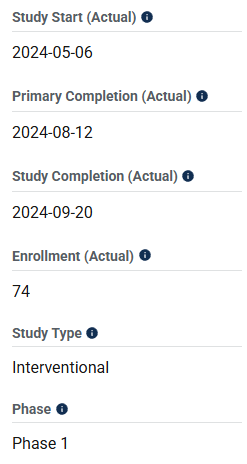
The trial was planned for 28 days, so early June 2024 would have been the absolute earliest Phase 1 data could have been finalized. Additionally, the Phase 2 study has its primary completion reported as February 27th, 2025: over a year after Mr. Tran’s statements on alleged Phase 2 adverse event rates.
There’s a lack of congruency in Biomed Industries’ research timelines. I believe that neither of these trials took place, and the studies, as well as claimed outcomes in press releases, journals and other forms of media, are fictitious. The submissions to ClinicalTrials.gov are filled with other pieces of evidence that cast doubt on the credibility of Biomed Industries and the existence of Bioglutide.
Small Molecule vs. Peptide (With Bonus Plagiarism)
Throughout many press releases and news articles, Biomed Industries has repeatedly referred to Bioglutide as a “small molecule drug”.
They did so in their initial news post in February 2024 (live, archived):
NA-931 is a small molecule drug available in oral formulation.
They did so in a press release from October of 2024 (live, archived):
NA-931, a small molecule quadruple receptor agonist, is being developed for the treatment of both type 2 diabetes and obesity.
And again in a press release from September of 2025 (live, archived):
NA 931, also known as Bioglutide™, is a small molecule medicine designed to activate four metabolic hormone receptors
Almost all GLP-1 RAs are peptides (orforglipron being a notable exception), which within the context of medicine, are categorically distinct from small molecules. Most orally active drugs classified as small molecule have a mass less than 500 daltons (Lipinski’s rule of five), whereas GLP-1 RA peptides tend to be far larger. For example, semaglutide’s molecular weight is 4,113.53 daltons and tirzepatide’s molecular weight is 4,813.53 daltons – an order of magnitude larger than most small molecule drugs. This isn’t a fuzzy boundary or spectrum: they’re mutually exclusive descriptors.
The first problem is Bioglutide’s branding: why did Biomed Industries opt to register a trademark with the -tide suffix (semaglutide and other GLP-1 RAs use this suffix because they’re peptides) if their drug is a small molecule? At best, it’s an intentionally misleading branding effort that piggybacks off of established weight loss drug names, but the bigger problem is that Biomed Industries itself isn’t quite sure what Bioglutide is (they also described it as a triple agonist, instead of a quadruple agonist, in March 2024 (live, archived)).
If we head back to the Phase 2 study (NCT06564753), Biomed Industries repeatedly refers to Bioglutide as a peptide:
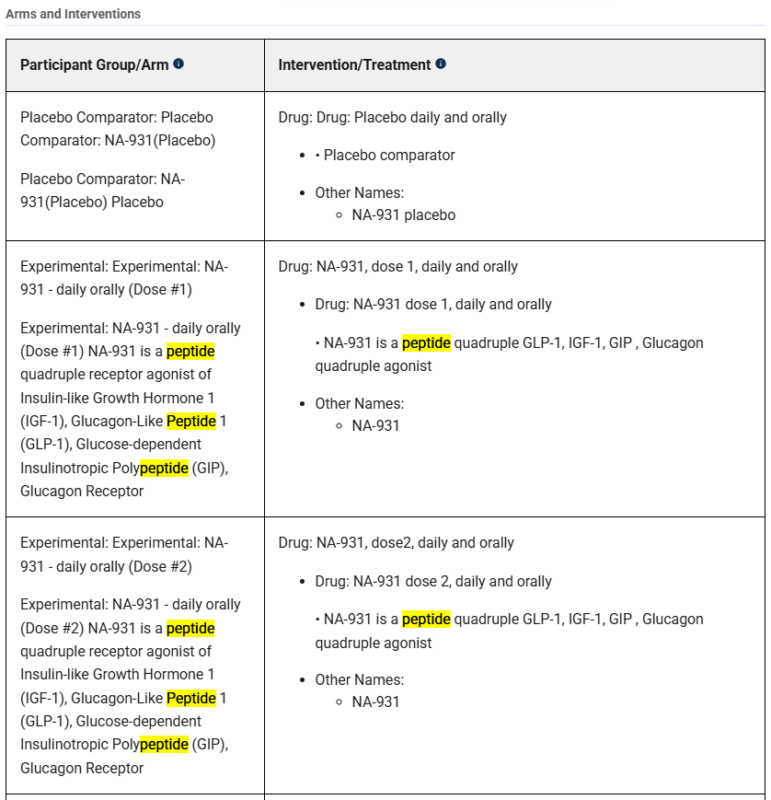
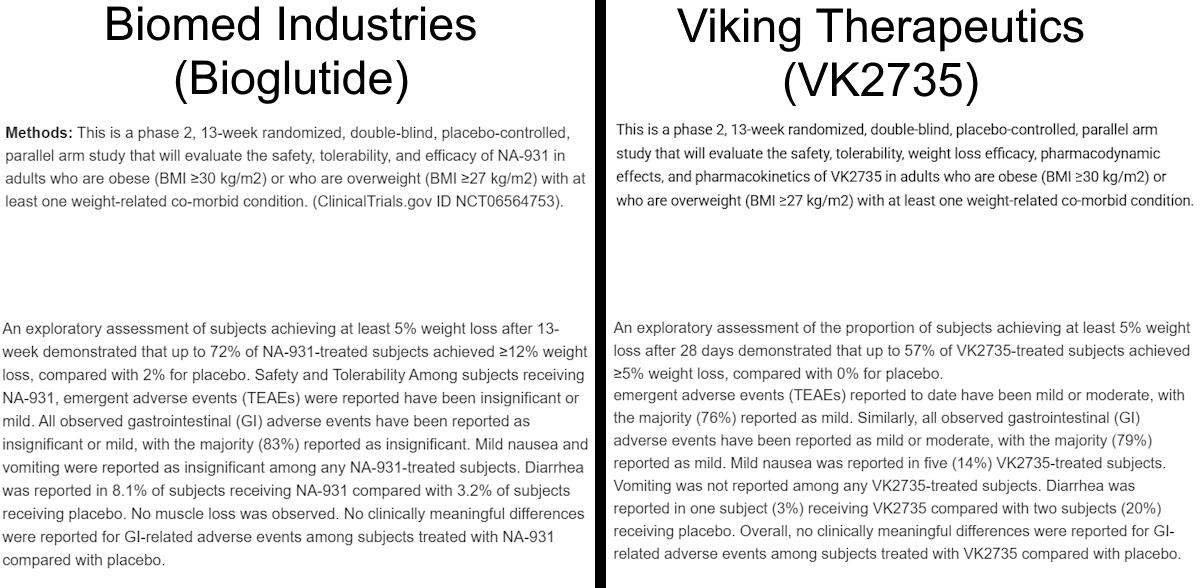
After more research, I found a simple explanation for this discrepancy: trial plagiarism. In the image above, you’ll see that there are repeated words and phrases at the start of various sections (Placebo Comparator, Drug, and Experimental). This is because almost all of the trial has been copy and pasted from an unrelated clinical trial. That trial was ran by Viking Therapeutics in August of 2023 for VK2735 (NCT06068946). VK2735 is a peptide dual agonist of GLP-1 and GIP (similar to tirzepatide). I encourage you to open both studies for yourself to compare them against one another:
It’s almost a word-for-word duplication, and I believe that Mr. Tran quite literally copy/pasted sections (which would explain the doubling of initial words and phrases in the above screenshot) to fill out Biomed Industries’ own trial. Here are a few examples of the similarities between the two studies:
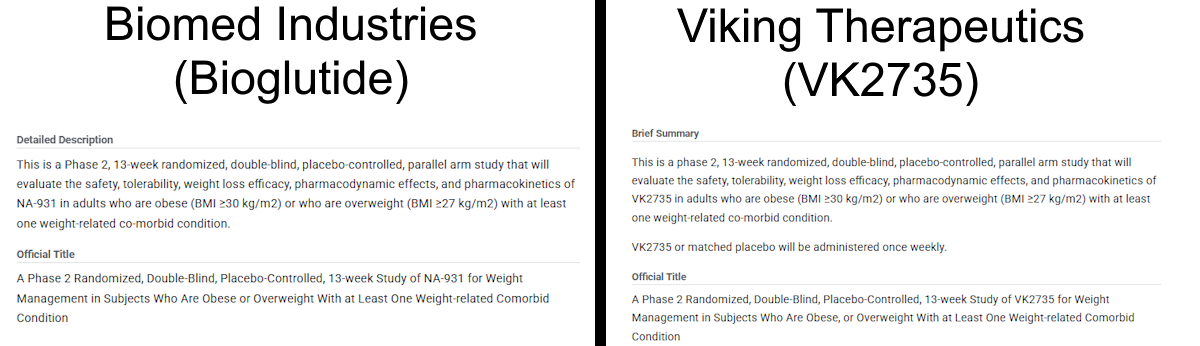 If we remove the small sentence starting with “VK2735 or matched placebo will be administered once weekly.”, the texts are essentially identical. The only differences are the drug name (VK2735 is replaced with NA-931) and the removal of a comma after “Obese” in the official title for Bioglutide’s study.
If we remove the small sentence starting with “VK2735 or matched placebo will be administered once weekly.”, the texts are essentially identical. The only differences are the drug name (VK2735 is replaced with NA-931) and the removal of a comma after “Obese” in the official title for Bioglutide’s study.
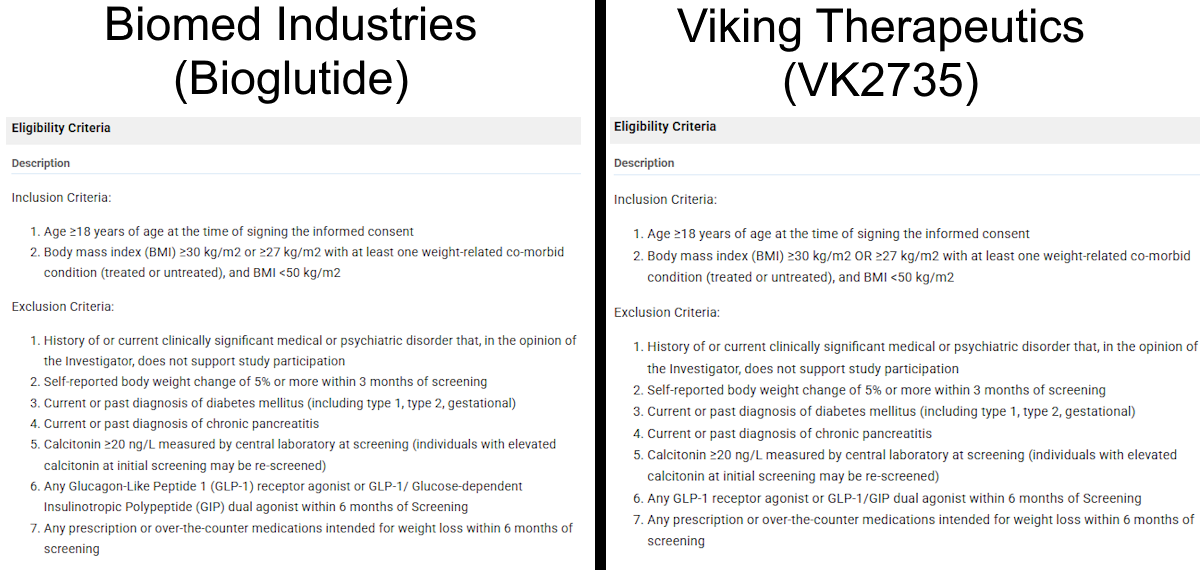 Aside from a small inclusion of GIP’s expanded name and the capitalization of “OR” in the second inclusion criteria, these two texts are identical.
Aside from a small inclusion of GIP’s expanded name and the capitalization of “OR” in the second inclusion criteria, these two texts are identical.
I believe Mr. Tran got lazy here: he copied the Arms and Interventions section and made some edits, but when he changed the information of VK2735 to Bioglutide, he neglected to remove the part that described VK2735 as a peptide, likely due to focusing on the switch of dual agonist to quadruple agonist and the additional information supplied.
There might be an alternative explanation, but it’s the most likely: a lazy oversight when copy and pasting text from a real pharmaceutical company’s clinical trial. The rest of the sections on the two trials also match, but I think I’ve shown enough to prove plagiarism here with the above examples. I don’t think endless screenshots proving text duplication is all that useful, but I found other studies over the years by Biomed Industries that had exactly the same pattern of clinical trial plagiarism. Biomed’s Phase 3 Oral Polio Vaccine study (NCT04540185) copies a huge amount of text from Moderna’s mRNA COVID vaccine (NCT04470427). The same is true for Biomed’s Bionetide study for Rett Syndrome (NCT06840496) which plagiarizes ACADIA Pharmaceuticals’ study of Trofinetide (NCT04181723). The latter example is particularly damming: just look at the primary and secondary outcome similarities. Biomed Industries’ plagiarism is beyond egregious and spans multiple years, multiple studies and multiple drugs.
Update: I also found Biomed Industries’ planned phase 2 study of NA-931 and Tirzepatide (NCT06732245) to be a carbon copy of Eli Lilly’s phase 2 study on Bimagrumab and Semaglutide (NCT05616013). Semaglutide was replaced with tirzepatide and Bimagrumab was replaced with NA-931. Mr. Tran was lazy enough to straight-up copy many of the testing site locations too. With the exception of the 3 California locations, all 14 of the others are listed in Eli Lilly’s trial documentation. Mr. Tran also included in exclusion criteria, “History of, or known hypersensitivity to, monoclonal antibody drugs”, which makes sense in Eli Lilly’s trial, because Bimagrumab is a monoclonal antibody. Bioglutide, on the other hand, isn’t – so this exclusion doesn’t make sense.
To sum this section up: I think Bioglutide has been intentionally misbranded with the -tide suffix and the reason why it was described as a peptide in the Phase 2 study was due to lazy plagiarism.
Bioglutide isn’t a peptide. Bioglutide isn’t a small molecule either, though: it doesn’t exist.
Mr. Tran’s June 2025 YouTube Interview
Mr. Tran held a ‘press conference’ after a presentation at the American Diabetes Association in June of 2025 and uploaded the video – unlisted – to his YouTube channel. In it, he talks about the alleged results of the Bioglutide Phase 2 trial. Here’s what Mr. Tran said about the racial background of the participants (please note: the background music is Mr. Tran’s addition, not mine):
In terms of race, about 60+ percent are white Caucasian, some Asian, some Afro-American, some Maori people in New Zealand, some Native American, so…
Again, if we look at the Phase 2 study’s data on ClinicalTrials.gov (NCT06564753), we see that only 5 locations were documented: all of them in Australia. How could Biomed Industries have recruited Maoris that were in New Zealand? I could potentially chalk this mistake up to English being Mr. Tran’s second language and what he actually meant to say was “Maori people from New Zealand”, but Australia’s Maori population is less than 1%, and I find it very hard to believe that in a study of just over 100 people, 15 Maoris were recruited alongside a non-zero number of Native Americans.
Biomed Industries has alleged to have conducted research in New Zealand before (NCT03538522), and they do have direct ties to an LLC in NZ (Bioneurals Limited), so my best guess is that Mr. Tran didn’t bother to check where Biomed Industries said they were conducting research for the Phase 2 study and, for whatever reason, decided to include Maoris on a whim.
I did consider going deeper into the New Zealand connections in this post, but I don’t think it’s required, and out of respect owing to a family member who passed away, I’ll leave this particular topic alone.
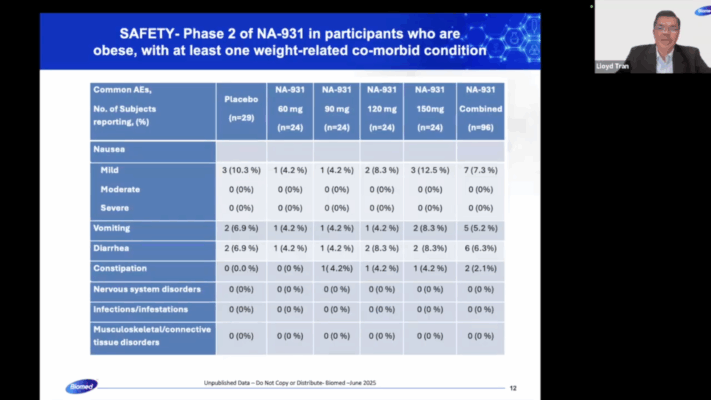
Elsewhere in the YouTube interview, Mr. Tran presents the following slides:
You may remember that Mr. Tran has previously plagiarized Viking Therapeutics and the clinical trial information related to VK2735: he’s done exactly the same thing in this presentation.
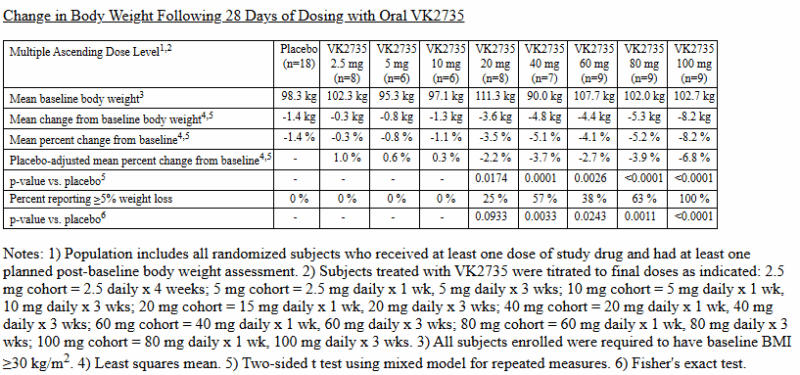
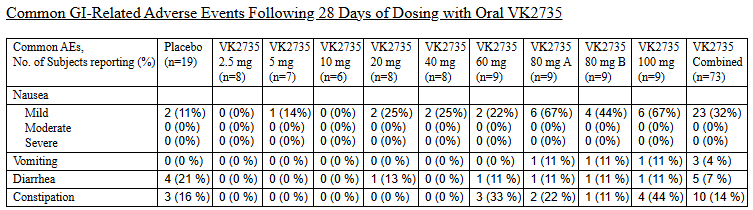
At ObesityWeek 2024, Viking Therapeutics released new data on their drug VK2735. This covered a 28-day oral dosing trial and the one that Mr. Tran plagiarized in Bioglutide’s Phase 2 ClinicalTrials.gov submission. The data for both is available here.
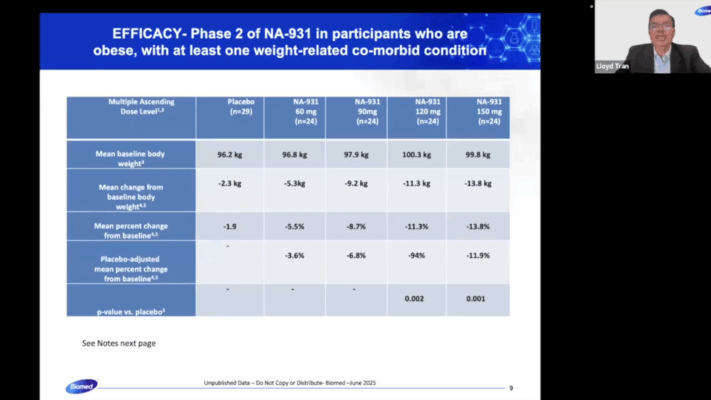
The formatting is unattractive, but there’s very clear plagiarism between the slides shown above and the data presented in this press release.
Note that for some reason, Biomed Industries appears to have preferentially copied the data structure and language from the 28-day trial instead of the 13-week trial (indicated by “Multiple Ascending Dose Level” instead of “Dose Level” and Note 1 beginning with “Population includes all…” instead of “Efficacy population…”.). I searched extensively for an article that matched the font, color scheme and style of the slides presented by Mr. Tran, but this was, unfortunately, a dead end.
Alas: there’s very clear and obvious plagiarism here – the data’s poorly presented too, with inconsistent structure (e.g. -5.3kg without a space, 1.9 not having a % sign and -94% not having a decimal point). Perhaps someone more experienced with fraudulent data analysis could pull apart Mr. Tran’s video and in particular, the demographic profile presented in the video snippet above. Again, I believe I’ve presented enough evidence here to show extensive fraud by Mr. Tran, so I shan’t venture too far out of my lane.
A few other points regarding the presentation: despite claiming many times that no lean mass loss occurred during Bioglutide’s Phase 2 trial, it’s not mentioned or presented in the data in any capacity. Completely ignored. None of the data they claimed to have gathered could have possibly verified lean mass preservation. Additionally, the supplement Mr. Tran authored and submitted to the Diabetes journal claimed:
reductions in mean body weight from baseline, up to 14.8 % at 150 mg daily dosage, or 13.2% % relative to placebo. [sic]
Which differs from the 13.8% change from baseline and 11.9% placebo-adjusted figure in the video.
Question: why do these figures differ, and why is the implied placebo figure 1.6% instead of the 1.9% shown?
Answer: plagiarism, and again, Mr. Tran is stealing from Viking Therapeutics and VK2735. Compare the submitted journal’s text above with this press release.
In particular, VK2735’s subcutaneous Phase 2 VENTURE trial: it reported a 14.7% reduction, or 13.1% relative to placebo. Mr. Tran simply increased both numbers by 0.1% for the Diabetes journal, but this data doesn’t align with the videos’, both in terms of the figures and placebo gap.
Other parts of the Diabetes submission are also plagiarized. The ‘Method’ section is the same verbatim theft observed from VK2735’s VENTURE study (NCT06068946), with the second section heavily lifted from VK’s pipeline page.
I have edited the visual structure slightly to assist with comparison, but the text was copied and pasted verbatim, and you’re encouraged to compare the two documents for yourself.
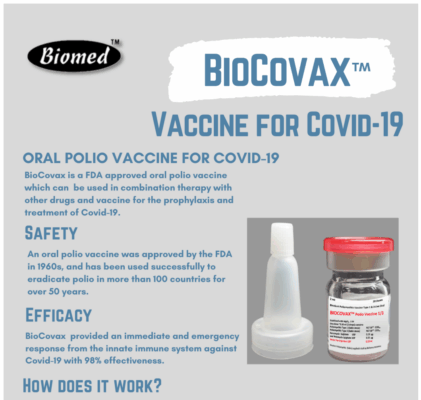
A Quick Look at BioCovax
I promise I have a smoking gun waiting for you in the next section, but I want to briefly cover BioCovax, which is described as follows by Biomed Industries (live, archived).
Biocovax is an oral vaccine comprised of oral polio vaccine and NA-831 for the prophylaxis and treatment of early onset of Covid-19 [sic]
Biomed Industries’ writing across many platforms is consistently poor: grammatical errors, spelling mistakes, sloppy formatting and so on – parsing this sentence is difficult, but the gist of it is that ‘BioCovax’ is just a generic oral polio vaccine that you take with their drug – NA-831 – to mitigate potential side effects. I’m not familiar enough with trademark law or medical product description requirements, but describing BioCovax as an oral vaccine – when it’s just a polio vaccine with a pill taken separately – is dishonest. This isn’t a novel vaccine worthy of a trademark.
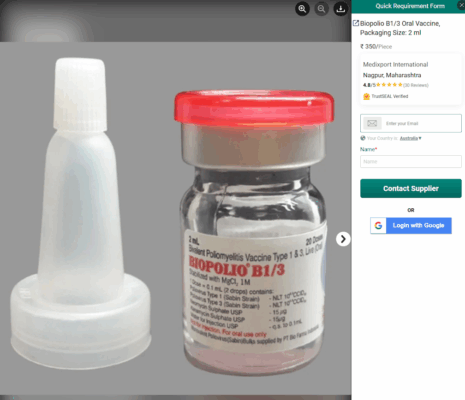
On the same page, Biomed Industries has a two-page product brochure that seems to pivot away from the inclusion of NA-831 and just references BioCovax as its own distinct product. A product image is suppled in this PDF, but the image has been stolen from a website that just sells the generic vaccine under the name BioPolio – although they’ve poorly photoshopped on a very low-resolution label to make it look like a product they’ve created.
On the product page, the following statement is made:
Clinical trials results have demonstrated that NA-831 or Traneurocin, a neuroprotective drug can be administered orally in conjunction with oral polio vaccine to mitigate any potential risks of vaccine-associated paralytic polio.
But if we look at the relevant ClinicalTrials.gov entry (NCT04540185), this clinical trial never received any update. Biomed Industries’ claim that clinical trial results have demonstrated NA-831’s ability to mitigate the risk of vaccine-associated paralytic polio is completely unsubstantiated. I cannot find any clinical trial results that authenticate this claim. I believe their 3,600 participant trial never took place and they’re fraudulently claiming to have trial results that demonstrate efficacy when no such results exist.
Google and the SEC
I don’t know who made the conscious decision to archive SEC news digests in 1991, or who prompted for those digests to be uploaded to the SEC’s website, but what I do know is that Google indexes those news digests, and on page 5 of Issue 91-212 dated November 1, 1991, the following entry exists:
I did my absolute best to find out more about this case, but I have limited resources, and while it might not be proof in isolation, it confirms all of my priors about Mr. Tran. 35 years ago, the current CEO of Biomed Industries sold shares in his medical company to over 100 investors and did so while making false and misleading statements, as well as omitting to state material facts. In today’s money, the $2 million he swindled out of investors amounts to around $4.8 million. According to another SEC digest, his company was declared bankrupt not long after this scheme. If you find out more about SEC v. Loi H. Tran, please let me know. Oh, and for posterity, it’s the same Lloyd H. Tran – he even lists CRT on his LinkedIn profile.
Bayes to the Rescue
My skepticism regarding the existence of Bioglutide (and all NA-branded drugs Mr. Tran claims to have invented, for that matter) can be neatly summarized as follows:
What are the odds that a man with a history of making false and misleading statements about his medical company discovers the most powerful anorectic drug known to man that would be worth tens, if not hundreds of billions of dollars, given its alleged effects?
Biomed Industries has never brought an NA-branded drug to market.
Biomed Industries has never completed a phase 3 trial.
Biomed Industries has repeatedly engaged in plagiarism.
Biomed Industries has made false claims about its drugs and their efficacy.
Bioglutide doesn’t exist, and I’d wager good money that over the next few years, Biomed Industries will attempt to attract investors and defraud them with its fake trials and fake data.
Questions for Biomed Industries
Despite my confidence and certainty of Biomed Industries engaging in wholesale scientific fraud, I could be wrong. In theory, Mr. Tran could defend his company – and his alleged discoveries – but there are serious questions that require serious answers. Here are questions that Biomed Industries must answer:
- When Bioglutide was announced in February 2024, Mr. Tran said: “the drug has shown only minor adverse events in a Phase 2A trial”. Bioglutide’s only Phase 2 trial submitted to ClinicalTrials.gov didn’t finish until February 2025. How did Mr. Tran have information on adverse event rates from a Phase 2 trial, even before Bioglutide’s Phase 1 trial had begun?
- Biomed Industries has consistently and repeatedly made references to Bioglutide’s alleged ability to preserve lean mass, but no study design or reported results have referenced data that would confer this fact either directly or indirectly. What trial data does Biomed Industries rely on to substantiate its lean mass preservation claims?
- In Bioglutide’s Phase 2 trial documentation (NCT06564753), it’s referred to as a peptide, but elsewhere, Biomed Industries has called it a small molecule. Why was Bioglutide described as a peptide in this submission?
- Biomed Industries’ NA-931 Phase 2 trial documentation (NCT06564753) has extensive similarities with VK2735’s Phase 2 trial documentation (NCT06068946). Does Biomed Industries acknowledge this plagiarism and do they have a reasonable explanation?
- Biomed Industries’ NA-831 Phase 3 trial documentation (NCT04540185) has extensive similarities with Moderna’s mRNA-1273 trial documentation (NCT04470427). Does Biomed Industries acknowledge this plagiarism and do they have a reasonable explanation?
- Biomed Industries’ NA-921 Phase 3 trial documentation (NCT06840496) has extensive similarities with ACADIA Pharmaceuticals’ Trofinetide trial documentation (NCT04181723). Does Biomed Industries acknowledge this plagiarism and do they have a reasonable explanation?
- Biomed Industries’ NA-931 Phase 2 with Tirzepatide trial documentation (NCT06732245) has extensive similarities with Eli Lilly’s Bimagrumab and Semaglutide trial documentation (NCT05616013). Does Biomed Industries acknowledge this plagiarism and do they have a reasonable explanation?
- In the June 2025 press conference video uploaded to Mr. Tran’s YouTube channel, he claimed that Biomed Industries had recruited Maori, Afro-American and Native American participants for its Phase 2 trial, but the trial documentation only shows 5 recruitment sites, all of which were in Australia. Were the 5 reported sites correct? If they were correct, was Mr. Tran’s racial breakdown correct? Why did Mr. Tran state that Maori participants were recruited in New Zealand? If the reported sites weren’t correct, what sites were used and why weren’t they accurately documented on the ClinicalTrials.gov submission?
- At any point, for any of Biomed Industries’ Phase 1, 2 or 3 trials, has any third-party clinical trial management service assisted with Biomed Industries’ research? If yes, can any verification from those third parties be provided?
- Mr. Tran’s submission to Diabetes (Supplement) has extensive similarities to Viking Therapeutics’ VK2735 press release data. Does Biomed Industries acknowledge this plagiarism and do they have a reasonable explanation?
- Mr. Tran’s submission to Diabetes (Supplement) claims that Bioglutide’s Phase 2 trial demonstrated weight loss of 14.8% and 13.2% relative to placebo in the 150mg cohort. In Mr. Tran’s press conference video, he shows slides that instead present the figures of 13.8% and 11.9%. What explains the differences between the values? Does Mr. Tran have any explanation for the similarities between the figures and those in Viking Therapeutics’ VK2735 trial data? (14.8% vs. 14.7% and 13.2% vs. 13.1%).
- Biomed Industries’ product page for BioCovax on their website states that “Clinical trials results have demonstrated that NA-831 or Traneurocin, a neuroprotective drug can be administered orally in conjunction with oral polio vaccine to mitigate any potential risks of vaccine-associated paralytic polio.”. What clinical trial demonstrated this? Did Biomed Industries’ Phase 3 study for NA-821 (NCT04540185) ever happen?
- In the June 2025 press conference video uploaded to Mr. Tran’s YouTube channel, the slides presented on Bioglutide regarding efficacy, notes and safety have extensive similarities to Viking Therapeutics’ VK2735 press release data. Does Biomed Industries acknowledge this plagiarism and do they have a reasonable explanation?
Those Involved
Sunlight is the best disinfectant, and I want to ensure that those involved in this con are appropriately identified so anyone who might involve themselves with these people in future can know that they are dishonest and deceitful.

Mr. Tran is Chairman and CEO of Biomed Industries, Inc. Previously owned Controlled Release Technologies, Inc. in the late 1980s and defrauded investors out of roughly $4.8 million USD in 2025 dollars. He’s likely the ‘mastermind’ behind the current fraud at Biomed Industries. Currently based in San Jose, California.

Mr. Tran is Vice President of Bioinformatics and AI at Biomed Industries, Inc. I believe him to be Lloyd’s brother and he’s just as complicit in the fraud. In another unlisted video available here, he talks extensively about the Phase 2 trial of Bioglutide and its results. Results he knows were faked. Listed as the principle investigator on the Phase 2 Bioglutide study (NCT06564753).

Michael Willis is Vice President of Business Development at Biomed Industries, Inc and CEO of MedAware, a subsidiary of Biomed Industries. Complicit in the fraud: talks about interest from investors in the June video, where he states:
It’s been very very busy over the last few weeks … we’ve had numerous meetings with these very large pharmaceutical companies. Companies like Novo Nordisk, Merc, Pfizer … I can keep naming names. AstraZenica approached us at this conference and they really want to start opening a dialog with us … we were the bell of the ball at this event.
Is listed as a testing facility contact across multiple sites in Biomed Industries’ NA-921 (Bionetide) trial that’s due to start at the end of October (NCT06840496).

Fern Vu is Vice President of Operations of Biomed Industries, Inc. and Lloyd Tran’s wife. Listed in some studies and on some documentation – likely has no active involvement, but well aware of her husband’s fraud.
I believe these 4 individuals are the only employees of Biomed Industries. Some studies have ‘Jennifer Thompson’ and ‘Jennifer Thomposn’ listed as a contact, but the phone number/email is shared with others at the firm and I was unable to find LinkedIn profiles, references in videos or anything else that would suggest she exists. Likely not a real person.
Conclusion
If you’ve invested in Biomed Industries, contact a lawyer immediately and submit a complaint to the SEC. If you’re in a Vietnamese community that may have been targeted by Mr. Tran, inform them. Feel free to translate this article in its entirety into Vietnamese.
If there are any notable updates, I’ll be sure to post them right here.









Great work! Can’t wait to see what happens next! Hopefully some news organizations will get wind of this and do some more investigative reporting to see what more can be dug up.
I work in this industry and want to add more another option to look into this/raise flags. WCG Center watch (clinical trial recruitment, but usually used only with studies using WCG as a central IRB), as they have this AUS phase 2 listed as still recruiting. It’s worth reaching out to WCG – in case they are the IRB of record. https://www.centerwatch.com/clinical-trials/listings/NCT06564753/phase-2-trials-of-na-931-to-study-subjects-who-are-obese-with-at-least-one-weight-related-comorbid-condition
Next, the historical version of the phase 2 study listing on clinicaltrials.gov has contacts for the study “sites” listed, except for all of the sites the site contacts listed are David Nguyen and Jennifer Thompson with biomed emails. I have never seen a sponsor list themselves as the contacts for all sites. Same for the US phase 2. Sponsors without specifically isolated recruiting teams should not be receiving patient contacts under any circumstances, and when they exist it goes to a central email and isn’t listed site level. Site contacts aren’t always listed at all but this is super unusual.
Also feel free to reach out to me. The Rhett syndrome studies also have the same site contact patterns, but as a rare disease is there are additional ways to find if it’s real.
This serves as a Cease and Desist notice from Biomed Industries, Inc. regarding this video. The video contains false statements and wrongful accusations that are defamatory and damaging to the reputation and business interests of Biomed Industries, Inc. (www.biomedind.com) .
You are hereby demanded to immediately remove and disable public access to this video within 48 hours of receipt of this notice.
Please be advised that if you fail to comply, Biomed Industries, Inc. will pursue all available legal remedies, including a multi-million-dollar lawsuit for defamation and related damages, without further notice.
On behalf of Biomed Industries, Inc.
You are hilarious. 😂
An Anonymous cease and desist? You couldn’t even come up with a name for a law firm? Don’t you have to serve papers or something?